(801) 782-9269
365 E Lomond View Dr STE 201 North Ogden, UT 84414
Inlays and Onlays in North Ogden
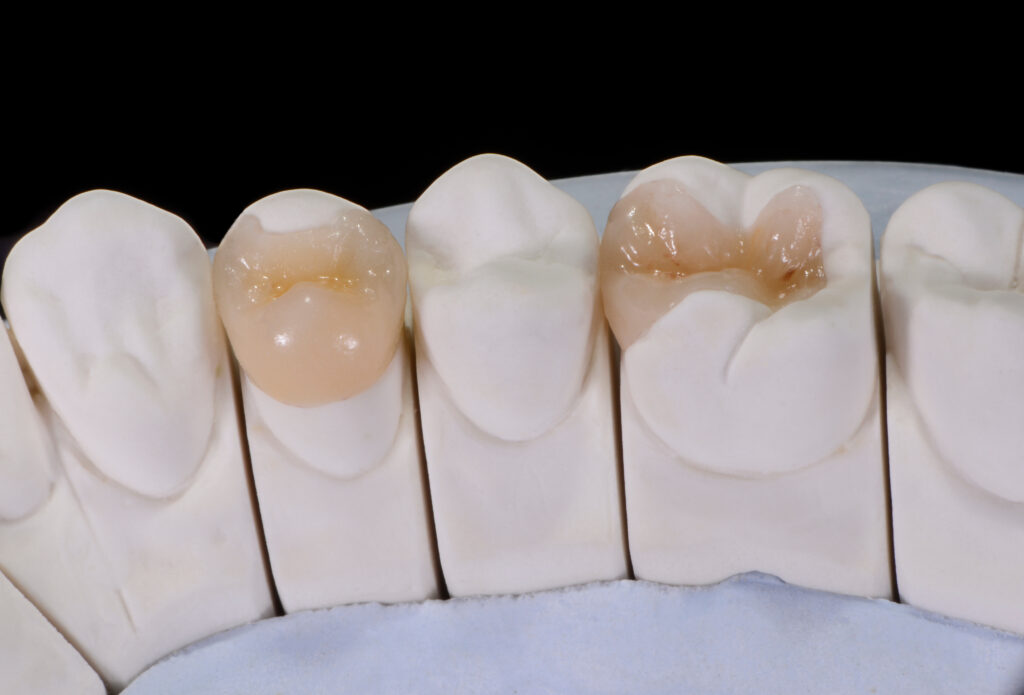
What are Inlays and Onlays?
What to Expect When Getting Inlays and Onlays
- The first step the dentist will take is putting a local anesthetic to numb the area.
- Then, the dentist will remove the decayed area of the tooth.
- The dentist will then take impressions of the teeth so that either the inlay or onlay will have the perfect fit to your tooth. The impression will be sent out for the permanent inlay or onlay to be made, while the temporary one will be made in the office.
- The next step will be to schedule a second appointment to fit your permanent inlay or onlay and cement it to the tooth.
- The last step is to shape and polish to make sure that the inlay or onlay is the perfect fit and looks like the natural tooth.
Common Concerns
Is the process of getting inlays and onlays painful?
Since the doctor will be administering a local anesthetic for the procedure, it is unlikely that you will feel pain during the procedure.
How long do inlays and onlays last?
Inlays and onlays should last for many years with proper oral care. Make sure that you attend your general checkups and cleaning to ensure that the inlays and onlays are in perfect condition and last for many years.
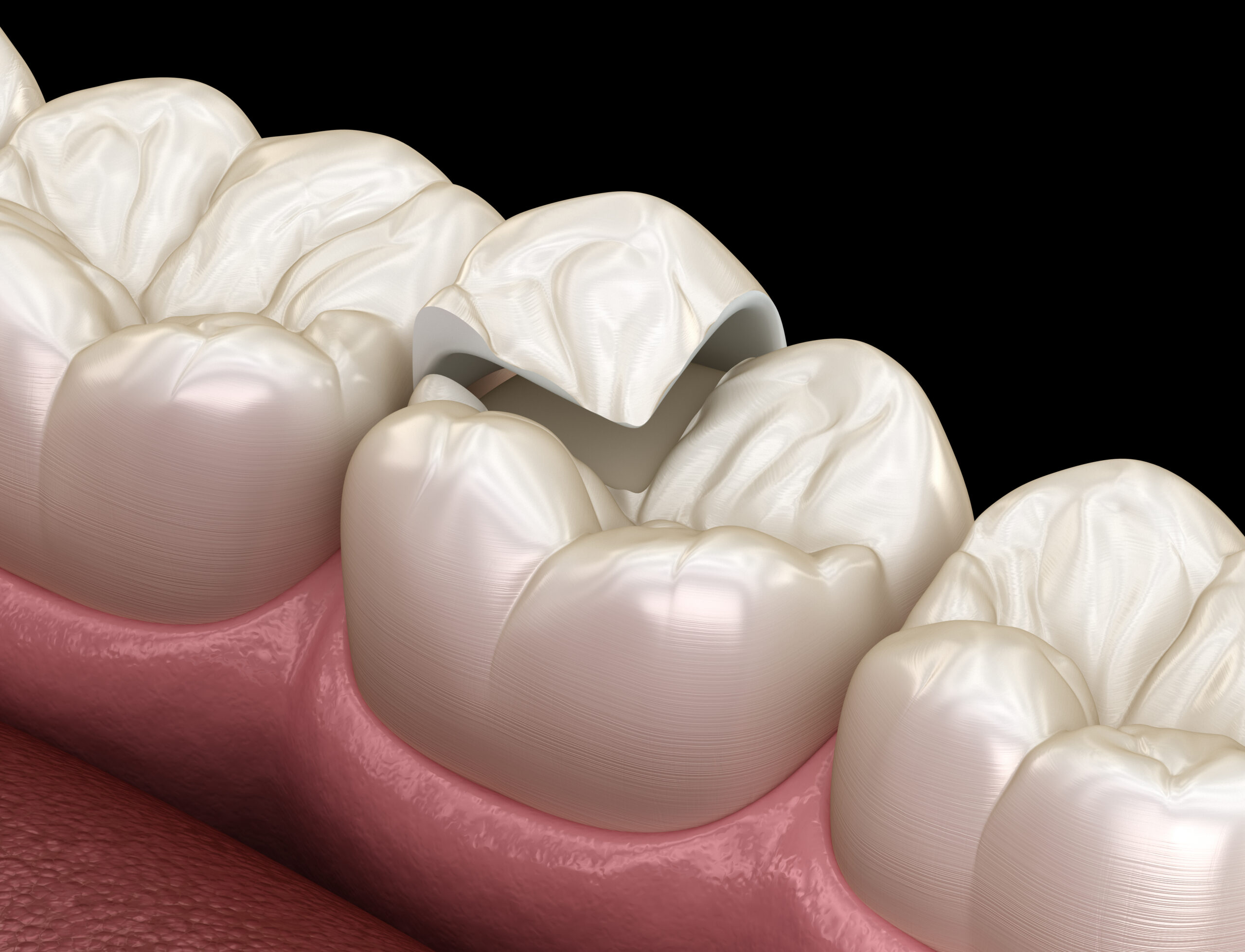
Inlays and onlays are the perfect way to enhance and protect your smile. Give our office a call to schedule an appointment and ask about inlays or onlays at (801) 782-9269.
Our Services:
Frequently Asked Questions
Yes. Inlays and Onlays can be used on the back and front teeth. They are a great natural solution for fixing decayed teeth.
Yes. After the procedure, you will be able to eat and drink as usual.
This will depend on the specific insurance provider and insurance plan. It’s best to give your insurance provider a call to get the exact details of the benefits. Our team will also be sure to help you answer any questions you may have about your benefits.
There is no special care for inlays and onlays. However, maintaining good oral health and having regular checkups and cleanings are recommended to ensure that the inlays or onlays are in perfect condition.
This would depend on each specific case. Sometimes, it is possible to get the inlays and onlays done on the same day. However, it is also possible that the procedure may require multiple appointments. Before treatment is started, our team will go over the treatment plan and make sure that we find the best option for you.
Yes. Inlays and onlays can replace the metal fillings. This will give a better option for matching the material to the natural tooth while also preventing further decay.
Inlays and onlays are made from a material that is less prone to staining compared to regular fillings. This helps keep the natural look of the tooth over a long period of time.
Yes, inlays and onlays are good alternatives to filling cavities. They provide a durable solution to restoring decayed teeth.
Yes. Our team is experienced in helping patients with dental anxiety feel comfortable from start to finish.
During the consultation with our team, the dentist will be sure to check your mouth and determine the right course of treatment for your specific needs.
BOOKING HOURS
M 12-7 | Tu 9:30-5 | Wed 8-5 | Th 7-5 | Fr 8-2
We are OPEN for ALL dental care procedures and emergency needs. Protecting the health and safety of our patients, families, and team members remains our number one priority.
Free $50 Gift Card*
$50 Gift Card when you complete an appointment with us!
*Redeemable after completed paid treatment. Must be a new patient our organization (all locations). The patient will receive a gift card via email or SMS after completion of their appointment. Cannot be combined with other offers or dental discount plans.
Free Exam & X-rays^
For New Patients without insurance we offer Free Exam and X-rays!
^For New Patients that do not have dental insurance. New patients must be 18 or older to receive free exam and x-rays. Discounts cannot be combined with other offers or dental discount plans. Additional fees may be included in individual cases.
$99 Hygiene Visit^^
For New Patients without insurance we offer Free Exam and X-rays!
^^For new patients without dental insurance. Includes Exam, X-Ray and Routine Cleaning. A Periodontal Cleaning requires additional fees, and rescheduled for further treatment. Cannot be combined with other offers or dental discount plans.

